All Features

William A. Levinson
The Pareto principle calls for focus on the vital few rather than the trivial many. While none of ISO 9001’s clauses are trivial—a nonconformance for any of them requires corrective action—ISO 9001 users can avoid most nonconformances by focusing on the clauses that are the most frequent trouble…

Laurel Thoennes @ Quality Digest
Compliance to U.S. Food and Drug Administration (FDA) regulations has come a long way in the past 30 years. Here are the main changes. Have they affected your business?
1988: Food and Drug Administration ActOfficially establishes the FDA as an agency of the Department of Health and Human Services…

Dirk Dusharme @ Quality Digest
As the United States struggles with rising healthcare costs, reducing the amount of money pharmaceutical companies spend dealing with regulation, while at the same time meeting drug safety requirements, would seem to be competing interests.
The goal of any honest pharmaceutical company is to make…

Graham Freeman
Many industries have no clear boundary between safety and quality culture. In fact, they are often closely integrated. Quality failures and nonconformances that require rework have been correlated with increased accidents and recordable injury rates in manufacturing organizations. These injuries…

Wendy White
Starting a new facility in the food-processing industry is an enormous undertaking. There are thousands of things that must be accomplished, from hiring and training new staff to ordering and installing equipment. This scenario is a perfect example of “too much to do and not enough time to do it…

Richard Harpster
On Oct. 13, 2018, the Automotive Industry Action Group (AIAG) sponsored a webinar on the status of the AIAG Core Tools Software (AIAG CTS). John Cachat, AIAG project manager for the AIAG CTS project, was the presenter for the webinar. The presentation provided information on why the AIAG was…

Dirk Dusharme @ Quality Digest
We tied up last year in a neat little bow, talking about how stories define ourselves and our work; waste is waste, no matter your political leanings; and putting numbers from the news in context.
“The Gift of Being Small” This article by Quality Digest’s Taran March wonderfully illustrates how we…

Bretta Kelly
Back in January 2009, I wrote an article for Quality Digest titled “ISO 9001 Documentation Is Like a Box of Chocolates.” Here we are, almost 10 years later, all of the ISO 9001 and related standards have been updated, yet companies still misunderstand what to document, how to document, or why to…
William A. Levinson
The U.S. government’s Fourth National Climate Assessment warns that climate change “creates new risks and exacerbates existing vulnerabilities in communities across the United States, presenting growing challenges to human health and safety, quality of life, and the rate of economic growth.” The…

NIST
A convocation of delegates representing 60 countries voted last month in Versailles, France, to implement the most significant change to the International System of Units (SI) in more than 130 years. For the first time, all measurement units will be defined by natural phenomena rather than by…

NIST
The U.S. Department of Commerce announced that the 2018 Malcolm Baldrige National Quality Award will be given to two educational institutions, an organ donor group, a hospital, and a project management firm. A presidential-level honor, the award recognizes exemplary U.S. organizations and…

Dirk Dusharme @ Quality Digest
In this episode we look at lessons learned (or not) from GE, the difference between ISO and FDA “requirements,” and this year's Baldridge recipients.
“GE’s Lessons Won’t Determine Whether You Succeed or Fail”
Does the success or failure of GE’s CEO really matter that much when it comes to how most…
Ryan E. Day
BioBridge Global (BBG) is a parent organization for four subsidiary organizations, three of which are involved in production activities, and they’re all around regenerative medicine, including blood components, clinical laboratory testing, and cell and tissue therapies. Organizations in the life…

Taran March @ Quality Digest
These days, even regulatory agencies must innovate if they expect to keep pace with the speed of doing business. The U.S. Food and Drug Administration is no exception, and this year especially it has challenged itself to find ways to enhance efficiency and update old regulations. Quality Digest has…

Dirk Dusharme @ Quality Digest
The Dec. 31, 2018 deadline looms for medical device companies that sell their devices in Canada. On that day, any company that sells medical devices to Canada will either need to hold an MDSAP certificate or show proof that they are on track to be MDSAP certified, or they won’t be able to sell…

Mike Richman
The future is the ultimate abstraction; anyone who has ever attempted to discern the nature of tomorrow by looking at the yesterdays leading up to today knows that prediction is a fool’s errand. That’s the unfortunate reality for weather forecasters, stockbrokers, sports bookmakers, political…
Ryan E. Day
One of the unique aspects of Finch Therapeutics is that although its product does not fall easily into any regulated category and thus is not FDA-approved, the company has been working closely with the agency for at least five years. The FDA has broad jurisdiction to regulate all health products,…

Matthew M. Lowe
Life science companies play a major role in the global economy, with revenues expected to reach a staggering $1.5 trillion by 2020.1 Such a rosy forecast is likely to attract innovators and encourage current industry players to blaze new trails. Whether new or established, life science companies…

William A. Levinson
Chad Kymal1 gave an excellent overview of the ISO 45001 occupational health and safety (OHS) standard that was released in March 2018. I purchased a copy of the standard, and it provides an excellent framework, modeled on Annex SL, which defines the structure of all the new ISO standards, for an…
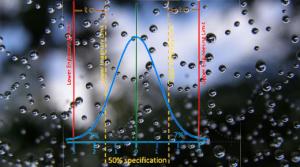
Anthony Chirico
In my first article, the merits and cautions of AS9138 c=0 sampling plans were discussed and a simple formula was provided to determine the required sample size to detect nonconforming units. In the second article, the process control properties of MIL-STD-105 c>0 sampling plans were…

Andreas Engelhardt
An international standard that specifies requirements for an occupational health and safety (OH&S) management system, ISO 45001:2018—“Occupational health and safety management systems–requirements” replaces OHSAS 18001 as the primary OH&S standard used internationally. It follows other…

Anthony Chirico
In my previous article, I discussed the merits and cautions of the “acceptance number” equal zero (c=0) sampling plans contained within AS9138. A simple formula was provided to determine appropriate sample size, and it was illustrated that twice the inspection does not provide twice the consumer…

Chad Kymal
Omnex began working in the automotive industry by assisting Ford powertrain suppliers in 1986. The U.S. automotive industry’s Big Three used GM’s Targets for Excellence, Ford’s Q 101, and Chrysler’s SQA standards to qualify its supply bases. The automotive industry was making deep reductions in its…

Anthony Chirico
Aerospace standard AS9138—“Quality management systems statistical product acceptance requirements” was issued this year (2018), a few years after its accompanying guidance materials in section 3.7 of the International Aerospace Quality Group’s (IAQG) Supply Chain Management Handbook. The new…

Mike Richman
IMTS was a blast, but it was great to be back home in lovely Northern California this week. On this episode of QDL, we covered the skills that workers need and the innovations that organizations want. Plus, we brought you a live interview with author Mark Graban, and one on tape from Burt Mason of…