All Features

Wendy Wood
Employers have a stake in their staff’s health. It’s not just a matter of keeping health insurance premiums in check which is a consideration in countries without universal healthcare. It’s also about maximizing employee engagement and productivity, and even happiness.
Promoting health habits is…

Michael Armstrong, Kenneth Klassen
Patients often wait weeks or months for medical appointments. Canada’s Fraser Institute recently reported that Canadians typically wait 10 weeks to see specialists. Long wait times are one reason Canada ranks behind other developed countries in healthcare quality.
In the United States, waits are…

Scott Gottlieb, Jeffrey Shuren
In recent days, the U.S. Food and Drug Administration (FDA) has committed to several new policies that will modernize the agency’s approach to regulation in the medical device system.
For instance, we announced our intention to propose an alternate approach to the traditional 510(k) clearance…

Rob Matheson
Liquid-liquid separation and chemical extraction are key processes in drug manufacturing and many other industries, including oil and gas, fragrances, food, wastewater filtration, and biotechnology.
Three years ago, MIT spinout Zaiput Flow Technologies launched a novel continuous-flow liquid-…

Jon Speer
Complaint handling continues to be one of the biggest reasons medical device companies receive 438s and warning letters from the U.S. Food and Drug Administration (FDA). Companies have a lot going on once a medical device has reached the market, and it can be challenging to keep up with…

Eric Cooper
You’re in the market to build a new house. Would you tell the builder what you’re looking for, or would you just tell him to build “something?” If the latter, what’s the likelihood that the house you end up with is going to be what you want? Documenting your requirements should be obvious, right…

Mike Richman
During last Friday’s episode of Quality Digest Live, we looked at the far-reaching implications of a prospective merger, previewed our latest webinar with DNV, considered the importance of fun at work, and inspected some interesting stereo microscopes from Vision Engineering. Here’s a closer look…

Sharona Hoffman
CVS operates 9,700 pharmacies and 1,000 MinuteClinics. A decade ago, it also purchased Caremark and now operates CVS/Caremark, a pharmacy benefits manager (PBM), a type of business that administers drug-benefit programs for health plans. CVS/Caremark is one of the three largest PBMs in the United…

Scott Gottlieb
Twice a year the federal government publishes the Unified Agenda of Federal Regulatory and Deregulatory Actions (Unified Agenda), which provides the American public with insight into regulations under development or review throughout the federal government. For the U.S. Food and Drug…

Jenna Gallegos, Jean Peccoud
Biology is becoming increasingly digitized. Researchers use computers to analyze DNA, operate lab equipment and store genetic information. But new capabilities also mean new risks, and biologists remain largely unaware of the potential vulnerabilities that come with digitizing biotechnology.
The…

Quality Transformation With David Schwinn
I was recently reminded of a fundamental statement about continual improvement. In Out of the Crisis (Massachusetts Institute Center for Advanced Engineering, 1986), W. Edwards Deming stated, “I should estimate that in my experience, most troubles and most possibilities for improvement add up to…

Lou Valdez, Dara Corrigan, Peter Stein
Regulatory experts from around the world, including the Food and Drug Administration (FDA), gathered recently to discuss issues such as regenerative medical products, international collaboration to fight antimicrobial resistance (AMR), and developing strategies to combat substandard or falsified…

Patrick Horine
Readmission of patients within 30 days of discharge is one of the most serious issues plaguing healthcare delivery in the United States. No one wants to go to the hospital, let alone return shortly after being discharged; readmissions also hurt hospital bottom lines.
Readmissions cost hospitals $…

Mike Richman
During last Friday’s episode of QDL, we examined the potential of quality thinking to improve outcomes for people’s health, manufacturing, and workplace efficiency. Let’s take a look:
“World Toilet Day” ISO truly has a standard (or at least a standard in development) for everything. World Toilet…

Jay Desai
The presidential symposium at this year’s annual meeting of the Child Neurology Society of America in early October in Kansas City raised many eyebrows: The first presentation focused on burnout rates among neurologists around the country.
Many of my colleagues felt that this was an inappropriate…
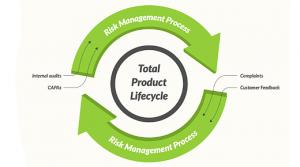
Jon Speer
What exactly is a risk-based quality management system (QMS)? This is a timely topic to get into. In 2016, ISO 13485—“Medical devices”—“Quality management systems” was updated, and one of the key concepts presented is the idea of a risk-based QMS.
Historically, regulations have almost exclusively…

Grant Ramaley
I have written previously about the Medical Device Single Audit Program (MDSAP) created by the International Medical Device Regulators Forum (IMDRF). MDSAP is viewed as a single audit covering the United States, Canada, Brazil, Australia, and Japan. The intent was to establish one medical-device…

Davis Balestracci
During the early 1990s, I was president of the Twin Cities Deming Forum. I had a wonderful board to work with, one of whom was Doug Augustine, our self-appointed provocateur. Doug was a 71-year-old retired Lutheran minister, and we all loved him because he always pulled us right back to earth with…

The QA Pharm
If I could summarize in one page the most important lessons I have learned in pharmaceutical quality assurance over the last 40 years, this is it.
When it comes to putting a procedure into written words, it doesn’t mean the words will be effective in getting people to follow the procedure.
The…

Dirk Dusharme @ Quality Digest
Our Oct. 27, 2017, episode of QDL looked at Ford, autonomous cars, and changes to FDA compassionate use rules.
“Ford Plans $14B in Cost Cuts as Part of New CEO’s Strategy”
Ford Motor Co’'s new CEO plans to cut $14 billion in costs, drop some car models, and focus the company’s resources on…
Scott Gottlieb
The FDA has a long history of supporting patient access to investigational new treatments. This includes working with drug and device companies through the clinical trial process that may lead to FDA approval of the treatment. We also offer expanded access programs that provide investigational…

Mike Richman
We cover a wide range of topics on QDL most weeks, but our latest episode, from Friday, Oct. 20, 2017, provided a steady drumbeat of technological detail. Here’s what we chatted about:
“Energy Harvested from Evaporation Could Power Much of U.S., Says Study” Renewal sources of energy like solar…

Ken Kingery
The first in-car measurements of exposure to pollutants that cause oxidative stress during rush-hour commutes has turned up potentially alarming results. The levels of some forms of harmful particulate matter inside car cabins was found to be twice as high as previously believed.
Most traffic…
Jon Speer
How confident are you when it comes to design validation? Does this always involve clinical evaluation, or not? We’ve found that, like many other terms in medical device development, the two can end up getting confused. When do you use one or the other?
There tends to be a lack of clarity out…

Dirk Dusharme @ Quality Digest
Our Oct. 13, 2017, episode of Quality Digest Live looked at edge computing for natural disasters, medical records, and zero defects.
“New Research May Improve Communications During Natural Disasters”
Could edge computing help communications during disasters?
“How Health Care Leaders Should…